This fits the usual profile of something I’d like to work on. It starts out really cheap, has a monstrous upside case but the catalyst remains far away, though a pretty obvious name to get some attention into pivotal data. Something like this only requires a couple positive opinions from the right people to see the stock really run up. However, now that it’s starting to do that, I question how deserved it is. I will say that I think there’s real upside here, probably the likes of which haven’t really been seen for a small-cap RCT since the SLNO readout. I don’t think that’s the right comparable in terms of ultimate magnitude (eventual 10x), but for shock factor/trading dynamic, and the idea that people might not even really be ready on what the stock should immediately be worth, that I could see. Something like 500m sales assumption with a 3x multiple would see the stock up around 300% from here, depending on where/how you want to slice the dilution. That’s probably a fine assumption, I just think the trial is pretty unlikely to work, which I’ll get into below. I’ve noticed some people saying they think the run-up trade is dead, I find this completely untrue. It’s maybe a bit more selective than when I first started, but this to me is still a prime example of that trade at work. Generally just requires either high POS or very high upside to work, instead of, you know, neither. Which is probably how it should be. I suspect in this case the upside is what’s appealing, rather than POS, but I digress.
ATYR’s proof of concept trial was interrupted by covid in 2020, confounding what was already a small dataset. Nobody’s really to blame for this obviously, things happen. But taking a small and hard to interpret dataset, and having it become smaller and harder to interpret, is unfortunate.
Of the 37 patients enrolled, 9 dropped out, so looking at a ~24% discontinuation rate, mostly for covid. There’s a few different ways to look at the data, so I’ll just link all of them here. You have the topline PR, slides, CHEST publication and supplement, and clinicaltrials.gov data.
They lead with the best data in the topline, as you’d expect:
58% steroid reduction, paired with lung symptom improvements in such a small trial, impressive! Let’s dig a little deeper.
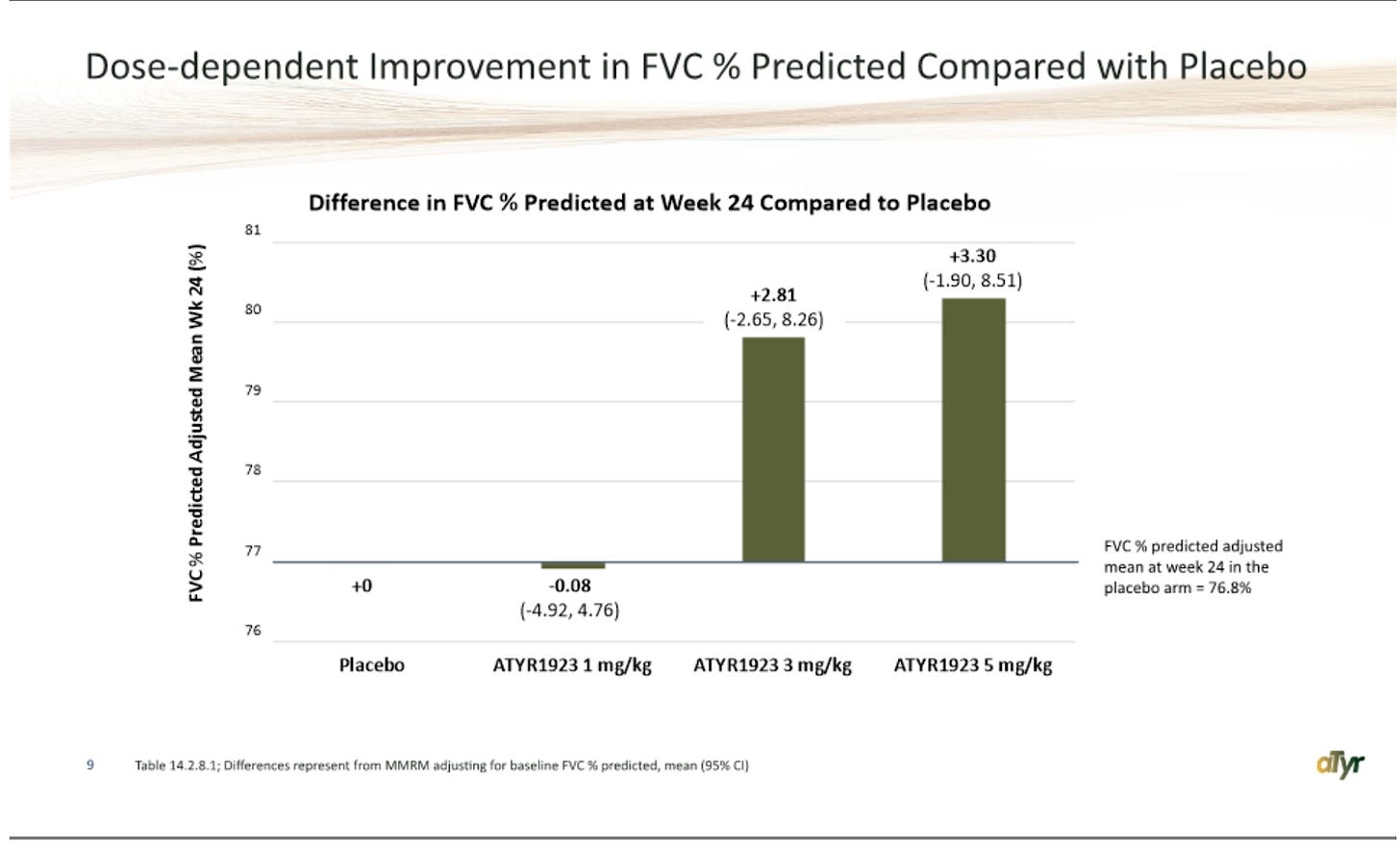
The 58% reduction they are referring to comes from the change in baseline mean, which, good news, I agree it makes the most sense to use! Unfortunately, it makes the rest of the data look worse. The low dose is worse on that endpoint, the middle dose is comparable, and the high dose looks mildly promising, coming in at 58% vs 46% for placebo, along with 3 patients tapered to 0 mg. I would argue that the proposed effect size is not impressive for having a third of the dose arm tapered -100% anyways. To me, this is somewhat reminiscent of another smaller cap longshot RCT in RPHM, where the debate revolved around how much stock you could put into a couple of hyper-responders, and the answer was none. Numerically, there’s a dose response here sure, but really it’s placebo/1mg/3mg looking extremely similar, and then 5 mg coming in a bit lower with a couple of strong outlier patients. The relative reduction versus placebo data looks a little better, I’d argue that again the 1 and 3 mg effect sizes aren’t meaningful (even the company has been saying they think >1 mg reduction would be meaningful for ph3), and so you end up with the same debate about interpreting potentially one active dose level with a couple of hyper-responders. The dropouts pose a problem for interpreting any of this data as is, and there’s *a chance* the high dose outliers are beneficiaries of being on drug, I just don’t think it’s likely in the context of the rest of the data.
Another way to look at the steroid data is shown on clinicaltrials.gov, patients who were able to taper to 5mg:
I cut the dataset so that you can see it all in one look, so it’s a little funny looking. The placebo arm had the most patients tapered to 5mg at week 24, and looks quite similar to 3 and 5 mg, while 1 mg looks much worse. Even if you give them the dropouts and subtract those from the n’s, they become (in order) 7/9, 1/6, 4/5, and 5/8. This makes 3 mg the best performing dose (barely), then placebo, the high dose performs almost 20% worse than 3mg, ~15% worse than placebo, and 1 mg is still really bad. So this is another way to look at the steroid data, I’d note the ways it was presented in the slides weren’t pre-specified on clinicaltrials.gov, but this was. I think the best reason to throw this data out would be to say that it’s uninterpretable given the dropouts and sample sizes, but that’s then obviously going to confound nearly every positive takeaway from the dataset as well…
I also find it interesting that their interpretation of this data was that they noticed they could “knock down steroids pretty well to 5 mg” because I don’t think that’s what that showed at all…
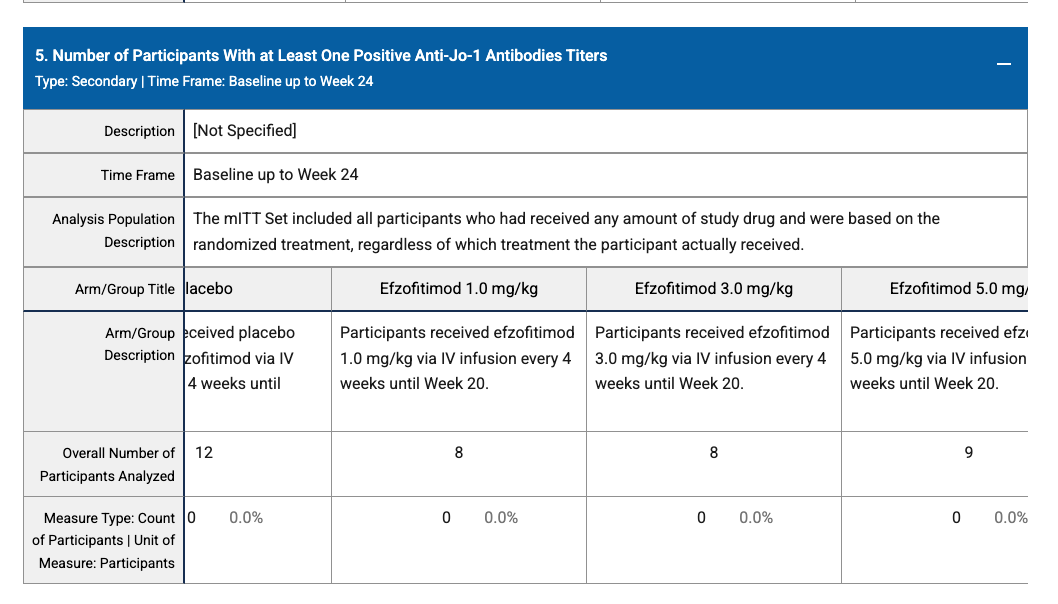
Some of the antibody data is confusing as well, more just that it doesn’t seem to demonstrate much of anything at all?
One placebo and one 3 mg responder?
And then zeroes across the board here? I’d be curious what they were trying to demonstrate with these, but I don’t see anything at all in this data. This is also relevant because I’d be curious to see some of the biomarker data here to get to what the drug is actually doing, they reference it in the topline:
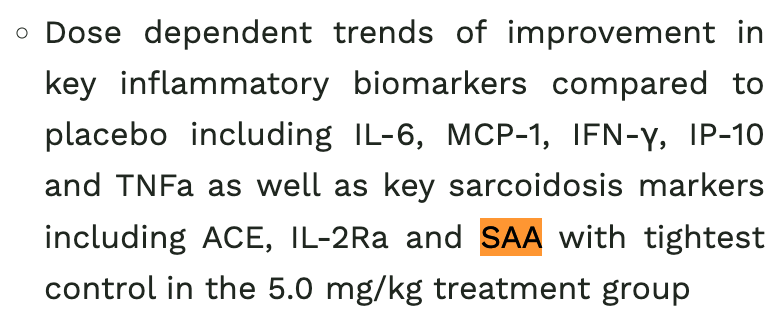
Dose dependent sure sounds great, but in the context of how they’ve interpreted some of the other data I would like to see it, and I can’t find it in any of the many data sources I linked. And it’s not referred to as stat sig, so presumably it’s not? SAA in particular would be interesting, because in the covid data they referred to it as the most important sarcoidosis biomarker, but there don’t seem to be slides filed with the data from that trial that show it either. This specifically is probably a non-issue, and the PR says it was stat sig in the covid trial, but I find the pre-specified steroid and antibody data a bit concerning.
Finally, there’s the lung symptom data to look at too:
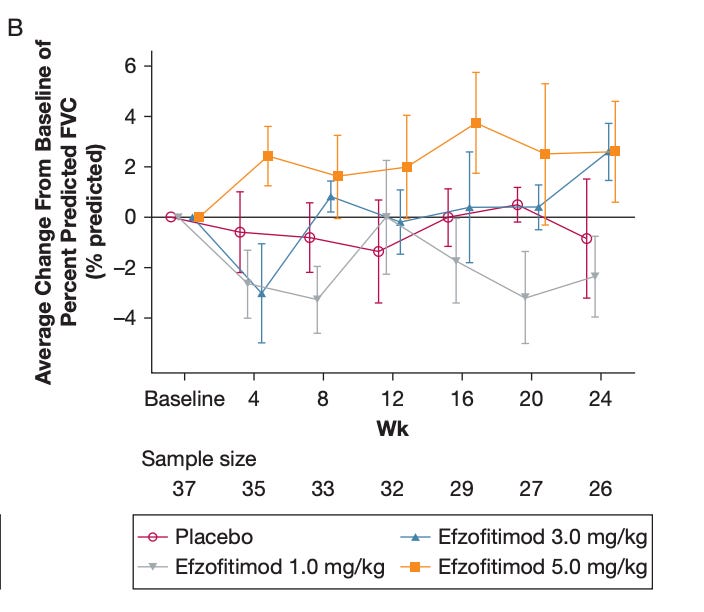
Impressive at the high dose here, and you see a similar improvement at 3 mg as well, and no change at the others. This is probably the best piece of evidence, but what would alarm me is that I think the 3 mg effect size is telling you this isn’t necessarily correlating with drug efficacy/steroid reduction. If patients on 3 mg are seeing drug efficacy, it sure didn’t show up in steroid reduction (only -3% pbo adj. change from baseline)… These are also huge CI’s, and again I’d caution against interpretability given dropouts, so it’s hard to take a ton away from any of this. But yes I would be concerned that these don’t clearly correlate at 3 mg, because that would call into question whether or not a similar 5 mg result matters all that much if 3 mg may have been achieved randomly. The idea that the drug would drive massive improvements at 3 mg specifically from weeks 4 to 8 and 20 to 24 doesn’t make any sense either, and doesn’t align with the 5 mg data.
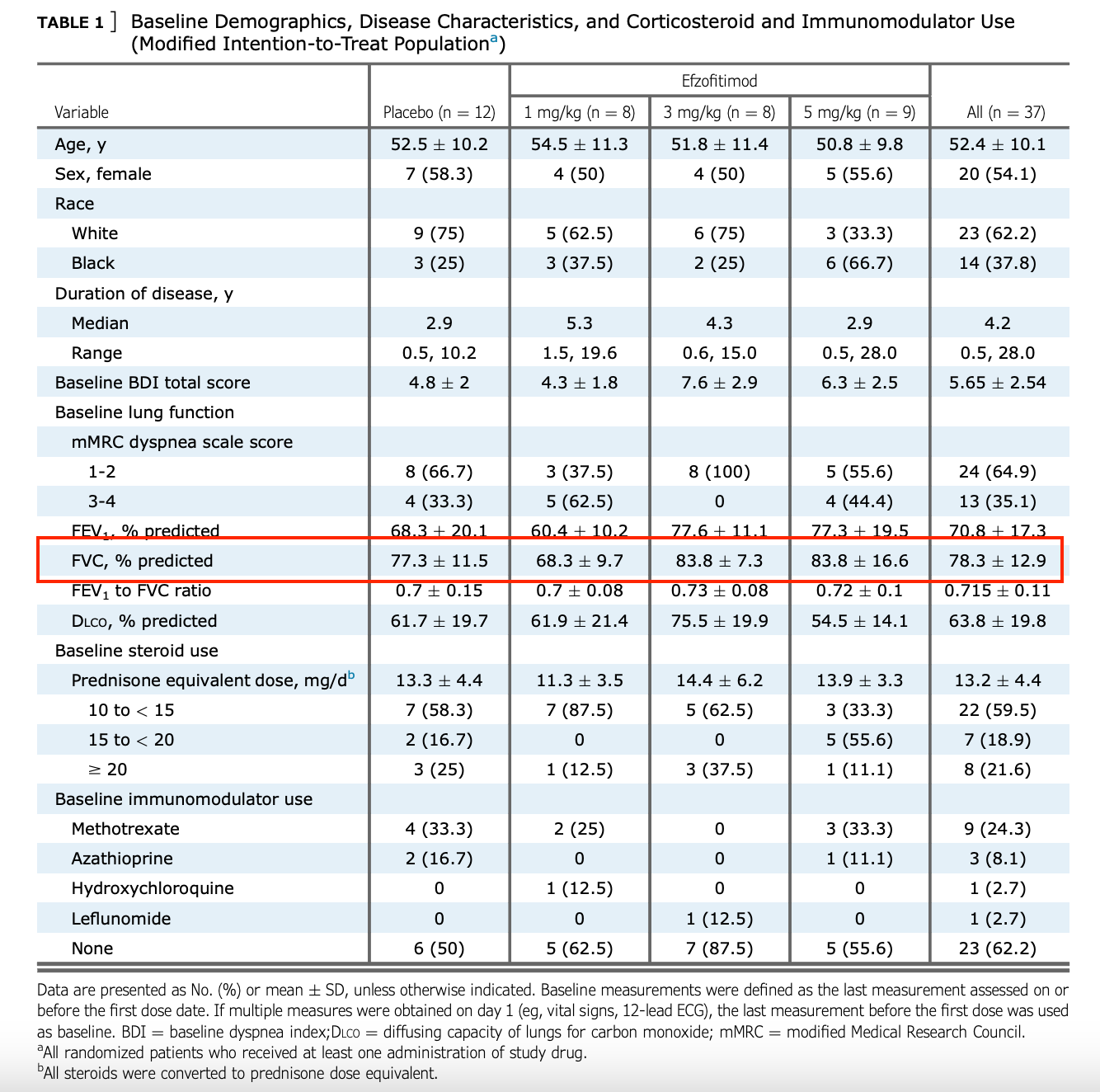
One possible explanation is that the healthiest patients by baseline FVC % were in the 3 and 5 mg groups:
It’s possible these patients are more likely to improve if starting from a higher baseline, that’d explain why the effect size is so similar despite different steroid reductions, just some food for thought.
I think what’s most interesting in reading up on this data and talking to people so far, views on this dataset are probably more based on how you individually are wired than any novel information or take. Everyone’s going to be looking at the same things here. You can talk to your KOLs about possible uptake, and upside targets will vary, but the POC data is the POC data. If you’re generally a more optimistic person, maybe this is enough for you to think it has some decent shot. If you’re not, it’s probably not. If you’re a sucker for the longshot RCT’s with a real upside tail, maybe you can still get the math to work at reasonably low odds. As for me, I expect this to fail, and to be able to structure a reasonable trade closer to data. Excited to see who’s right!









https://www.reddit.com/r/ATYR_Alpha/ the bull case
Can you comment on their claim that they have several different end points for the drug to pass FDA scrutiny, such as equivalency to steroids? And what do you think about their claim that subjects experienced quality of life improvements to the point that some wanted to stay on the drug (assuming that’s supported by the King’s sarcoidosis scores)? Thanks!